TOKYO and CAMBRIDGE, Mass., June 10, 2024 - (JCN Newswire) - Eisai Co., Ltd. and Biogen Inc. announced today that that the U.S. Food and Drug Administration (FDA) has accepted Eisai’s Supplemental Biologics License Application (sBLA) for monthly lecanemab-irmb (U.S. brand name: LEQEMBI®) intravenous (IV) maintenance dosing. A Prescription Drug User Fee Act (PDUFA) action date is set for January 25, 2025. LEQEMBI is indicated for the treatment of Alzheimer's disease (AD) in patients with mild cognitive impairment or mild dementia stage of disease (collectively referred to as early AD).
As part of the proposed monthly IV maintenance regimen, the patients who have completed the biweekly IV initiation phase, exact period under discussion with the FDA, would receive a monthly IV dose that maintains effective drug concentration to sustain the clearance of highly toxic protofibrils* which can continue to cause neuronal injury. The sBLA is based on modeling of observed data from the Phase 2 study (Study 201) and its open-label extension (OLE) as well as the Clarity AD study (Study 301) and its OLE study.
AD is a progressive disease caused by toxic amyloid proteins. Once established, this pathophysiological process continues through the patient’s life and therefore sustained treatment may be necessary. In those who are eligible, treatment should be initiated after diagnosis as early as possible to maximize patient outcomes. Data from Studies 201, 301 and their OLEs show that continued treatment with LEQEMBI beyond the 18-month core phase prolongs the benefit as highly toxic protofibrils are continuously removed. If the sBLA is approved, the clinical and biomarker benefits may be maintained through the once-monthly dosing regimen that is less burdensome and easier for patients and care partners to continue long-term.
Additionally, Eisai initiated the rolling submission of a BLA to the FDA for the LEQEMBI subcutaneous autoinjector for weekly maintenance dosing after it was granted Fast Track designation by the FDA in May 2024.
LEQEMBI is now approved in the U.S., Japan, China and South Korea, and applications have been submitted for review in the European Union, Australia, Brazil, Canada, Hong Kong, Great Britain, India, Israel, Russia, Saudi Arabia, Taiwan, Singapore, and Switzerland.
Eisai serves as the lead for lecanemab’s development and regulatory submissions globally with both Eisai and Biogen co-commercializing and co-promoting the product and Eisai having final decision-making authority.
* Protofibrils are believed to contribute to the brain injury that occurs with AD and are considered to be the most toxic form of Aβ, having a primary role in the cognitive decline associated with this progressive, debilitating condition.1 Protofibrils cause injury to neurons in the brain, which in turn, can negatively impact cognitive function via multiple mechanisms, not only increasing the development of insoluble Aβ plaques but also increasing direct damage to brain cell membranes and the connections that transmit signals between nerve cells or nerve cells and other cells. It is believed the reduction of protofibrils may prevent the progression of AD by reducing damage to neurons in the brain and cognitive dysfunction.2
INDICATION
LEQEMBI® [(lecanemab-irmb) 100 mg/mL injection for intravenous use] is indicated for the treatment of Alzheimer’s disease (AD). Treatment with LEQEMBI should be initiated in patients with mild cognitive impairment (MCI) or mild dementia stage of disease, the population in which treatment was initiated in clinical trials.
CONTRAINDICATION
LEQEMBI is contraindicated in patients with serious hypersensitivity to lecanemab-irmb or to any of the excipients of LEQEMBI. Reactions have included angioedema and anaphylaxis.
WARNINGS AND PRECAUTIONS
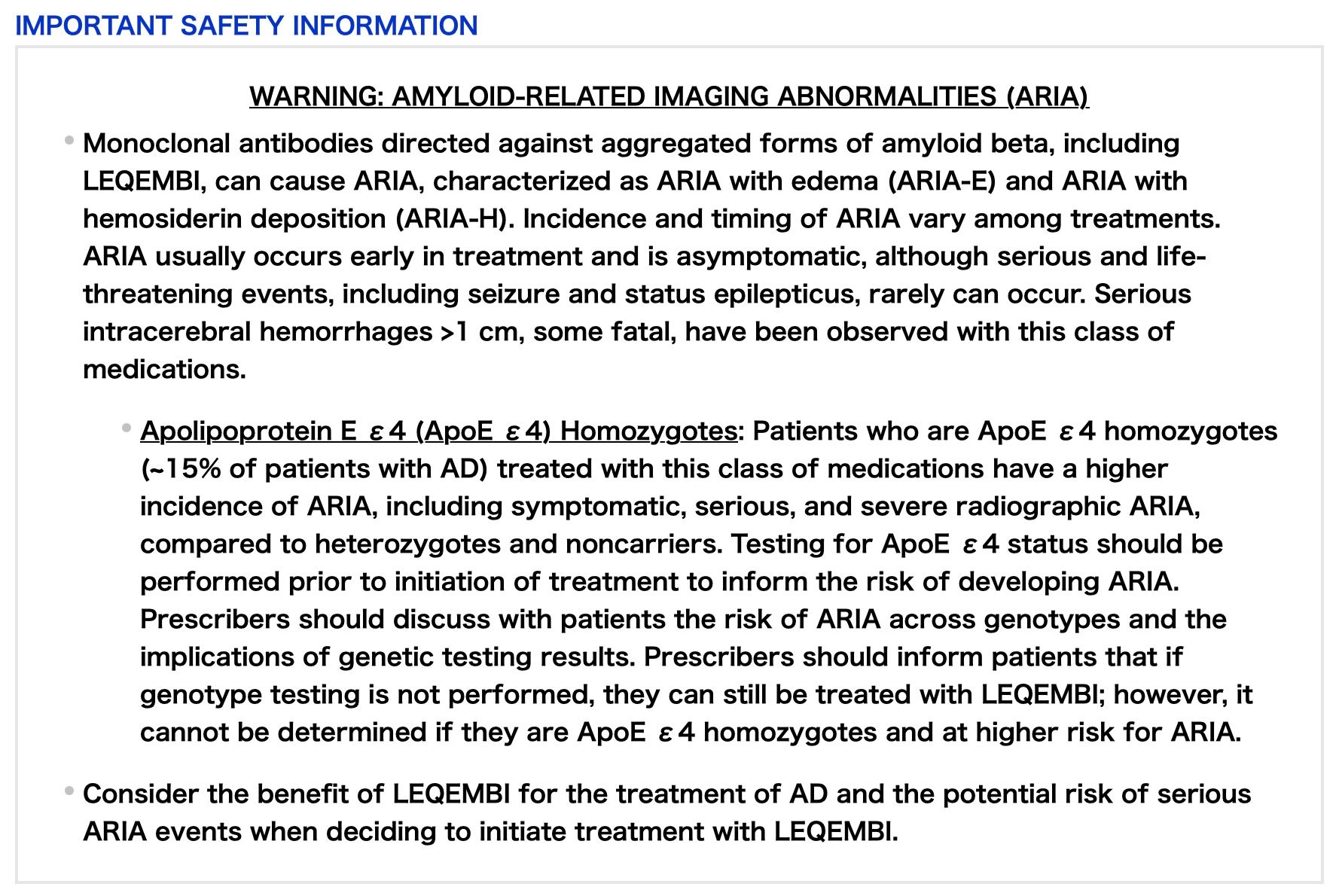
AMYLOID-RELATED IMAGING ABNORMALITIES
LEQEMBI can cause ARIA-E and ARIA-H, which can occur together. ARIA-E can be observed on magnetic resonance imaging (MRI) as brain edema or sulcal effusions and ARIA-H as microhemorrhage and superficial siderosis. ARIA can occur spontaneously in patients with AD. With this class of medications, ARIA-H generally occurs in association with ARIA-E. Reported ARIA symptoms may include headache, confusion, visual changes, dizziness, nausea, and gait difficulty. Focal neurologic deficits may also occur. Symptoms usually resolve over time.
Incidence of ARIA
Symptomatic ARIA occurred in 3% (29/898) and serious ARIA symptoms in 0.7% (6/898) with LEQEMBI. Clinical ARIA symptoms resolved in 79% (23/29) of patients during the period of observation. ARIA, including asymptomatic radiographic events, was observed: LEQEMBI, 21% (191/898); placebo, 9% (84/897). ARIA-E was observed: LEQEMBI, 13% (113/898); placebo, 2% (15/897). ARIA-H was observed: LEQEMBI, 17% (152/898); placebo, 9% (80/897). No increase in isolated ARIA-H was observed for LEQEMBI vs placebo.
ApoE ε4 Carrier Status and Risk of ARIA
Of the patients taking LEQEMBI, 16% (141/898) were ApoE ε4 homozygotes, 53% (479/898) were heterozygotes, and 31% (278/898) were noncarriers. With LEQEMBI, the incidence of ARIA was higher in ApoE ε4 homozygotes (LEQEMBI: 45%; placebo: 22%) than in heterozygotes (LEQEMBI: 19%; placebo: 9%) and noncarriers (LEQEMBI: 13%; placebo: 4%). Symptomatic ARIA-E occurred in 9% of ApoE ε4 homozygotes vs 2% of heterozygotes and 1% of noncarriers. Serious ARIA events occurred in 3% of ApoE ε4 homozygotes and in ~1% of heterozygotes and noncarriers. The recommendations on management of ARIA do not differ between ApoE ε4 carriers and noncarriers.
Radiographic Findings
The majority of ARIA-E radiographic events occurred within the first 7 doses, although ARIA can occur at any time, and patients can have >1 episode. Maximum radiographic severity of ARIA-E with LEQEMBI was mild in 4% (37/898), moderate in 7% (66/898), and severe in 1% (9/898) of patients. Resolution of ARIA-E on MRI occurred in 52% of patients by 12 weeks, 81% by 17 weeks, and 100% overall after detection. Maximum radiographic severity of ARIA-H microhemorrhage with LEQEMBI was mild in 9% (79/898), moderate in 2% (19/898), and severe in 3% (28/898) of patients; superficial siderosis was mild in 4% (38/898), moderate in 1% (8/898), and severe in 0.4% (4/898) of patients. With LEQEMBI, the rate of severe radiographic ARIA-E was highest in ApoE ε4 homozygotes (5%; 7/141) vs heterozygotes (0.4%; 2/479) or noncarriers (0%; 0/278). With LEQEMBI, the rate of severe radiographic ARIA-H was highest in ApoE ε4 homozygotes (13.5%; 19/141) vs heterozygotes (2.1%; 10/479) or noncarriers (1.1%; 3/278).
Intracerebral Hemorrhage
Intracerebral hemorrhage >1 cm in diameter was reported in 0.7% (6/898) with LEQEMBI vs 0.1% (1/897) with placebo. Fatal events of intracerebral hemorrhage in patients taking LEQEMBI have been reported.
Concomitant Antithrombotic Medication: In Clarity AD, baseline use of antithrombotic medication (aspirin, other antiplatelets, or anticoagulants) was allowed if the patient was on a stable dose. The majority of exposures to antithrombotic medications were to aspirin. Antithrombotic medications did not increase the risk of ARIA with LEQEMBI. The incidence of intracerebral hemorrhage was 0.9% (3/328) in patients taking LEQEMBI with a concomitant antithrombotic medication at the time of the event vs 0.6% (3/545) in those who did not receive an antithrombotic. Patients taking LEQEMBI with an anticoagulant alone or combined with an antiplatelet medication or aspirin had an incidence of intracerebral hemorrhage of 2.5% (2/79) vs none in patients receiving placebo. Caution should be exercised when considering the administration of anticoagulants or a thrombolytic agent (e.g., tissue plasminogen activator) to a patient already being treated with LEQEMBI.Other Risk Factors for Intracerebral Hemorrhage:
Patients were excluded from enrollment in Clarity AD for findings on neuroimaging that indicated an increased risk for intracerebral hemorrhage. These included findings suggestive of cerebral amyloid angiopathy (prior cerebral hemorrhage >1 cm in greatest diameter, >4 microhemorrhages, superficial siderosis, vasogenic edema) or other lesions (aneurysm, vascular malformation). The presence of an ApoE ε4 allele is also associated with cerebral amyloid angiopathy. Caution should be exercised when considering the use of LEQEMBI in patients with factors that indicate an increased risk for intracerebral hemorrhage and in patients who need to be on anticoagulant therapy.
ARIA Monitoring and Dose Management Guidelines
Obtain a recent baseline brain MRI prior to initiating treatment with LEQEMBI and prior to the 5th, 7th, and 14th infusions. Enhanced clinical vigilance for ARIA is recommended during the first 14 weeks of treatment with LEQEMBI. Depending on ARIA-E and ARIA-H clinical symptoms and radiographic severity, use clinical judgment when considering whether to continue dosing or to temporarily or permanently discontinue LEQEMBI. If a patient experiences ARIA symptoms, clinical evaluation should be performed, including MRI if indicated. If ARIA is observed on MRI, careful clinical evaluation should be performed prior to continuing treatment.
HYPERSENSITIVITY REACTIONS
Hypersensitivity reactions, including angioedema, bronchospasm, and anaphylaxis, have occurred with LEQEMBI. Promptly discontinue the infusion upon the first observation of any signs or symptoms consistent with a hypersensitivity reaction and initiate appropriate therapy.
INFUSION-RELATED REACTIONS (IRRs)
IRRs were observed—LEQEMBI: 26% (237/898); placebo: 7% (66/897)—and the majority of cases with LEQEMBI (75%, 178/237) occurred with the first infusion. IRRs were mostly mild (69%) or moderate (28%) in severity. IRRs resulted in discontinuation of LEQEMBI in 1% (12/898). Symptoms of IRRs included fever and flu-like symptoms (chills, generalized aches, feeling shaky, and joint pain), nausea, vomiting, hypotension, hypertension, and oxygen desaturation.
In the event of an IRR, the infusion rate may be reduced or the infusion may be discontinued and appropriate therapy initiated as clinically indicated. Consider prophylactic treatment prior to future infusions with antihistamines, acetaminophen, nonsteroidal anti-inflammatory drugs, or corticosteroids.
ADVERSE REACTIONS
The most common adverse reaction leading to discontinuation of LEQEMBI was ARIA-H microhemorrhages that led to discontinuation in 2% (15/898) with LEQEMBI vs <1% (1/897) with placebo.
The most common adverse reactions reported in ≥5% with LEQEMBI (N=898) and ≥2% higher than placebo (N=897) were IRRs (LEQEMBI: 26%; placebo: 7%), ARIA-H (LEQEMBI: 14%; placebo: 8%), ARIA-E (LEQEMBI: 13%; placebo: 2%), headache (LEQEMBI: 11%; placebo: 8%), superficial siderosis of central nervous system (LEQEMBI: 6%; placebo: 3%), rash (LEQEMBI: 6%; placebo: 4%), and nausea/vomiting (LEQEMBI: 6%; placebo: 4%).
Please see full Prescribing Information for LEQEMBI, including Boxed WARNING.
About lecanemab (LEQEMBI®)
Lecanemab is the result of a strategic research alliance between Eisai and BioArctic. It is a humanized immunoglobulin gamma 1 (IgG1) monoclonal antibody directed against aggregated soluble (protofibril) and insoluble forms of amyloid-beta (Aβ).3 Lecanemab is approved in the U.S.,4 Japan,5 China6 and South Korea7. In the U.S., Japan, China and South Korea, the indications are as follows.
- U.S.: For the treatment of Alzheimer’s disease (AD). It should be initiated in patients with MCI or mild dementia stage of disease.4
- Japan: For slowing progression of MCI and mild dementia due to AD.5
- China: For the treatment of MCI due to AD and mild AD dementia.6
- South Korea: For the treatment in adult patients with MCI due to AD or Mild AD.7
LEQEMBI’s FDA approval was based on Phase 3 data from Eisai’s, global Clarity AD clinical trial, in which it met its primary endpoint and all key secondary endpoints with statistically significant results.3 The primary endpoint was the global cognitive and functional scale, Clinical Dementia Rating Sum of Boxes (CDR-SB). In the Clarity AD clinical trial, treatment with lecanemab reduced clinical decline on CDR-SB by 27% at 18 months compared to placebo.8 The mean CDR-SB score at baseline was approximately 3.2 in both groups. The adjusted least-squares mean change from baseline at 18 months was 1.21 with lecanemab and 1.66 with placebo (difference, −0.45; 95% confidence interval [CI], −0.67 to −0.23; P<0.001).8 In addition, the secondary endpoint from the AD Cooperative Study-Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS-MCI-ADL), which measures information provided by people caring for patients with AD, noted a statistically significant benefit of 37% compared to placebo.8 The adjusted mean change from baseline at 18 months in the ADCS-MCI-ADL score was −3.5 in the lecanemab group and −5.5 in the placebo group (difference, 2.0; 95% CI, 1.2 to 2.8; P<0.001).8 The ADCS MCI-ADL assesses the ability of patients to function independently, including being able to dress, feed themselves and participate in community activities. The most common adverse events (>10%) in the lecanemab group were infusion reactions, ARIA-H (combined cerebral microhemorrhages, cerebral macrohemorrhages, and superficial siderosis), ARIA-E (edema/effusion), headache, and fall.8
Eisai has also submitted applications for approval of lecanemab in 13 countries and regions, including the European Union (EU).
Since July 2020 the Phase 3 clinical study (AHEAD 3-45) for individuals with preclinical AD, meaning they are clinically normal and have intermediate or elevated levels of amyloid in their brains, is ongoing. AHEAD 3-45 is conducted as a public-private partnership between the Alzheimer's Clinical Trial Consortium that provides the infrastructure for academic clinical trials in AD and related dementias in the U.S, funded by the National Institute on Aging, part of the National Institutes of Health, Eisai and Biogen. Since January 2022, the Tau NexGen clinical study for Dominantly Inherited AD (DIAD), that is conducted by Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU), led by Washington University School of Medicine in St. Louis, is ongoing and includes lecanemab as the backbone anti-amyloid therapy.
About the Collaboration between Eisai and Biogen for AD
Eisai and Biogen have been collaborating on the joint development and commercialization of AD treatments since 2014. Eisai serves as the lead of lecanemab development and regulatory submissions globally with both companies co-commercializing and co-promoting the product and Eisai having final decision-making authority.
About the Collaboration between Eisai and BioArctic for AD
Since 2005, Eisai and BioArctic have had a long-term collaboration regarding the development and commercialization of AD treatments. Eisai obtained the global rights to study, develop, manufacture and market lecanemab for the treatment of AD pursuant to an agreement with BioArctic in December 2007. The development and commercialization agreement on the antibody lecanemab back-up was signed in May 2015.
About Eisai Co., Ltd.
Eisai's Corporate Concept is "to give first thought to patients and people in the daily living domain, and to increase the benefits that health care provides." Under this Concept (also known as human health care (hhc) Concept), we aim to effectively achieve social good in the form of relieving anxiety over health and reducing health disparities. With a global network of R&D facilities, manufacturing sites and marketing subsidiaries, we strive to create and deliver innovative products to target diseases with high unmet medical needs, with a particular focus in our strategic areas of Neurology and Oncology.
In addition, we demonstrate our commitment to the elimination of neglected tropical diseases (NTDs), which is a target (3.3) of the United Nations Sustainable Development Goals (SDGs), by working on various activities together with global partners.
For more information about Eisai, please visit www.eisai.com (for global headquarters: Eisai Co., Ltd.), and connect with us on X, LinkedIn and Facebook. For audiences based in the UK and Europe, please visit www.eisai.eu and Eisai EMEA LinkedIn.
About Biogen
Founded in 1978, Biogen is a leading biotechnology company that pioneers innovative science to deliver new medicines to transform patients’ lives and to create value for shareholders and our communities. We apply deep understanding of human biology and leverage different modalities to advance first-in-class treatments or therapies that deliver superior outcomes. Our approach is to take bold risks, balanced with return on investment to deliver long-term growth.
The company routinely posts information that may be important to investors on its website at www.biogen.com. Follow Biogen on social media – Facebook, LinkedIn, X, YouTube
References
1. Amin L, Harris DA. Aβ receptors specifically recognize molecular features displayed by fibril ends and neurotoxic oligomers. Nat Commun. 2021;12:3451. doi:10.1038/s41467-021-23507-z
2. Ono K, Tsuji M. Protofibrils of Amyloid-beta are Important Targets of a Disease-Modifying Approach for Alzheimer's Disease. Int J Mol Sci. 2020;21(3):952. doi: 10.3390/ijms21030952. PMID: 32023927; PMCID: PMC7037706.
3. LEQEMBI. Prescribing information. Eisai Inc. 2023.
4. US Food and Drug Administration. FDA Grants Accelerated Approval for Alzheimer’s Disease Treatment. Available at: https://tinyurl.com/ysckp838. Last accessed: March 2024.
5. Eisai Global. 2023. “LEQEMBI® Intravenous Infusion” (Lecanemab) Approved for the Treatment of Alzheimer’s Disease in Japan Available at: https://www.eisai.com/news/2023/news202359.html. Last accessed: March 2024.
6. Eisai Global. 2024. “LEQEMBI®” (Lecanemab) Approved for the Treatment of Alzheimer’s Disease in China. Available at: https://www.eisai.com/news/2024/news202403.html. Last accessed: March 2024.
7. Eisai Global. 2024. “LEQEMBI®” (Lecanemab) Approved for the Treatment of Alzheimer’s Disease in South Korea Available at: https://www.eisai.com/news/2024/news202436.html Last accessed: May 2024
8. van Dyck, H., et al. Lecanemab in Early Alzheimer’s Disease. New England Journal of Medicine. 2023;388:9-21. https://www.nejm.org/doi/full/10.1056/NEJMoa2212948.
Media Contacts:
Eisai
Eisai Co., Ltd.
Public Relations Department
TEL: +81 (0)3-3817-5120
Eisai Inc. (U.S.)
Julie Edelman
1-862-213-5915
Julie_Edelman@eisai.com
Eisai Europe, Ltd.
EMEA Communications Department
+44 (0) 786 601 1272
Emea-comms@eisai.net
Biogen
Biogen Inc.
Jack Cox
+ 1-781-464-3260
public.affairs@biogen.com
INVESTOR CONTACTS
Eisai Co., Ltd.
Investor Relations Department
TEL: +81 (0) 3-3817-5122
Biogen Inc.
Chuck Triano
+ 1-781-464-2442
IR@biogen.com
Biogen Safe Harbor
This news release contains forward-looking statements, about the potential clinical effects of lecanemab; the potential benefits, safety and efficacy of lecanemab; potential regulatory discussions, submissions and approvals and the timing thereof; the treatment of Alzheimer's disease; the anticipated benefits and potential of Biogen's collaboration arrangements with Eisai; the potential of Biogen's commercial business and pipeline programs, including lecanemab; and risks and uncertainties associated with drug development and commercialization. These statements may be identified by words such as "aim," "anticipate," "believe," "could," "estimate," "expect," "forecast," "intend," "may," "plan," "possible," "potential," "will," "would" and other words and terms of similar meaning. Drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. Results in early-stage clinical studies may not be indicative of full results or results from later stage or larger scale clinical studies and do not ensure regulatory approval. You should not place undue reliance on these statements.
These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements, including without limitation unexpected concerns that may arise from additional data, analysis or results obtained during clinical studies; the occurrence of adverse safety events; risks of unexpected costs or delays; the risk of other unexpected hurdles; regulatory submissions may take longer or be more difficult to complete than expected; regulatory authorities may require additional information or further studies, or may fail or refuse to approve or may delay approval of Biogen's drug candidates, including lecanemab; actual timing and content of submissions to and decisions made by the regulatory authorities regarding lecanemab; uncertainty of success in the development and potential commercialization of lecanemab; failure to protect and enforce Biogen's data, intellectual property and other proprietary rights and uncertainties relating to intellectual property claims and challenges; product liability claims; and third party collaboration risks, results of operations and financial condition. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from Biogen's expectations in any forward-looking statement. Investors should consider this cautionary statement as well as the risk factors identified in Biogen's most recent annual or quarterly report and in other reports Biogen has filed with the U.S. Securities and Exchange Commission. These statements speak only as of the date of this news release. Biogen does not undertake any obligation to publicly update any forward-looking statements.